



Next: 8.3.6 Conclusion
Up: Studies of Electron-Molecule
Previous: Intel Machines
The distributed-memory SMC program has been applied to a number of elastic
and inelastic electron-molecule scattering problems, emphasizing polyatomic
gases of interest in low-temperature plasma applications [JTIS:88a],
[Manos:89a]. Initial applications [Hipes:90a],
[Winstead:91d]
on the Mark IIIfp were to elastic scattering by ethylene (C H
H ), ethane
(C
), ethane
(C H
H ), propane (C
), propane (C H
H ), disilane (Si
), disilane (Si H
H ), germane
(GeH
), germane
(GeH ), and tetrafluorosilane (SiF
), and tetrafluorosilane (SiF ). We have since studied elastic
scattering by other systems, including phosphine (PH
). We have since studied elastic
scattering by other systems, including phosphine (PH ), propylene
(C
), propylene
(C H
H ) and its isomer cyclopropane, n-butane (C
) and its isomer cyclopropane, n-butane (C H
H ), and
1,2-trans-difluoroethylene, both on the Mark IIIfp and on the Intel
machines. We have also examined inelastic collisions with ethylene
[Sun:92a], formaldehyde (CH
), and
1,2-trans-difluoroethylene, both on the Mark IIIfp and on the Intel
machines. We have also examined inelastic collisions with ethylene
[Sun:92a], formaldehyde (CH O), methane (CH
O), methane (CH ), and silane
(SiH
), and silane
(SiH ). Below we present selected results of these calculations, where
possible comparing to experimental data.
). Below we present selected results of these calculations, where
possible comparing to experimental data.
Figure 8.10 shows integral elastic cross sections-that is,
cross sections summed over all angles of scattering, plotted as a function of
the electron's kinetic energy-for the two C H
H isomers, cyclopropane
and propylene. Scattering from propylene requires some special
consideration, because of its small dipole moment [Winstead:92a]. These
calculations were performed in the static-exchange approximation, neglecting
polarization and excitation effects, on 256-node partitions of the Delta.
The results in Figure 8.10 should be considered preliminary,
since studies to test convergence of the cross
section with respect to basis set are in progress, but we do not expect
major changes at the energies shown. Corresponding experimental values
have not been reported, but the total scattering cross section, of
which elastic scattering is the dominant component, has been measured
[Floeder:85a], [Nishimura:91a], and these data are included
in Figure 8.10. Both the calculation and the measurements
show a clear isomer effect in the vicinity of the broad maximum, which
gradually lessens at higher energies. At the level of approximation
(static-exchange) used in these calculations, the maxima in the cross
sections are expected to appear shifted to higher energies and
somewhat broadened and lowered in intensity. Thus, for propylene, where
some discrepancy is seen between the two measurements, our calculation
appears to support the larger values of [Nishimura:91a].
isomers, cyclopropane
and propylene. Scattering from propylene requires some special
consideration, because of its small dipole moment [Winstead:92a]. These
calculations were performed in the static-exchange approximation, neglecting
polarization and excitation effects, on 256-node partitions of the Delta.
The results in Figure 8.10 should be considered preliminary,
since studies to test convergence of the cross
section with respect to basis set are in progress, but we do not expect
major changes at the energies shown. Corresponding experimental values
have not been reported, but the total scattering cross section, of
which elastic scattering is the dominant component, has been measured
[Floeder:85a], [Nishimura:91a], and these data are included
in Figure 8.10. Both the calculation and the measurements
show a clear isomer effect in the vicinity of the broad maximum, which
gradually lessens at higher energies. At the level of approximation
(static-exchange) used in these calculations, the maxima in the cross
sections are expected to appear shifted to higher energies and
somewhat broadened and lowered in intensity. Thus, for propylene, where
some discrepancy is seen between the two measurements, our calculation
appears to support the larger values of [Nishimura:91a].
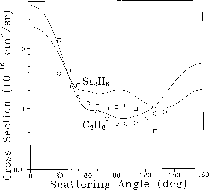
Figure 8.11: Differential Cross Sections for Elastic Scattering of  Electrons by Disilane and Ethane. Experimental points for ethane (circles)
are from Ref. [Tanaka:88a]; disilane data (squares) are from Ref.
[Tanaka:89a].
Electrons by Disilane and Ethane. Experimental points for ethane (circles)
are from Ref. [Tanaka:88a]; disilane data (squares) are from Ref.
[Tanaka:89a].
Figure 8.11 shows the plotting of the calculated
differential cross section, that is, the cross section as a function of
scattering angle, for elastic scattering of 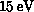 electrons from
ethane and its analogue disilane. These results were obtained on the
Mark IIIfp within the static-approximation. Agreement with experiment
[Tanaka:88a;89a], is quite
good; although there are quantitative differences where the magnitude
of the cross section is small, the qualitative features are well
reproduced for both molecules.
electrons from
ethane and its analogue disilane. These results were obtained on the
Mark IIIfp within the static-approximation. Agreement with experiment
[Tanaka:88a;89a], is quite
good; although there are quantitative differences where the magnitude
of the cross section is small, the qualitative features are well
reproduced for both molecules.
Calculations of electronic excitation cross sections are shown in
Figures 8.12 and 8.13. In
Figure 8.12, we present the integral cross section for
excitation of the 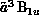 state of ethylene
[Sun:92a], obtained on the Mark IIIfp in a two-channel approximation.
This
state of ethylene
[Sun:92a], obtained on the Mark IIIfp in a two-channel approximation.
This 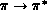 excitation weakens the C-C bond, and its cross
section is relevant to the dissociation of ethylene by low-energy electron
impact. As seen in the figure, the cross section increases rapidly from
threshold (experimental value
excitation weakens the C-C bond, and its cross
section is relevant to the dissociation of ethylene by low-energy electron
impact. As seen in the figure, the cross section increases rapidly from
threshold (experimental value 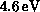 ) and reaches a fairly high peak
value before beginning a gradual decline. The threshold rise is largely due
to a d-wave (
) and reaches a fairly high peak
value before beginning a gradual decline. The threshold rise is largely due
to a d-wave (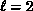 ) contribution, seen as a shoulder around
) contribution, seen as a shoulder around
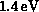 above threshold, which may arise from a core-excited shape
resonance. Relative measurements of this cross section [Veen:76a],
which we have placed on an absolute scale by normalizing to our calculated
value at the broad maximum, show a much sharper structure near threshold, but
are otherwise in good agreement.
above threshold, which may arise from a core-excited shape
resonance. Relative measurements of this cross section [Veen:76a],
which we have placed on an absolute scale by normalizing to our calculated
value at the broad maximum, show a much sharper structure near threshold, but
are otherwise in good agreement.
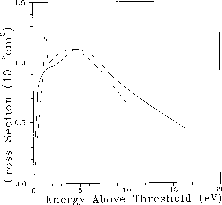
Figure 8.12: Integral Cross Section for Electron-Impact Excitation of the
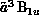 State of Ethylene. Solid line: present
two-channel result; dashed line: relative measurement of Ref. [Veen:76a],
normalized to the calculated value at the broad maximum.
State of Ethylene. Solid line: present
two-channel result; dashed line: relative measurement of Ref. [Veen:76a],
normalized to the calculated value at the broad maximum.
Figure 8.13 shows the cross section for electron-impact
excitation of the 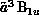 and
and 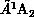 states of formaldehyde, obtained from a three-channel calculation. Portions
of this calculation were done on the Mark IIIfp, the iPSC/860, and the Delta.
Experimental data for these excitations are not available, but an independent
calculation at a similar level of theory has been reported
[Rescigno:90a], and is shown in the figure. Since the complex-Kohn
calculation of [Rescigno:90a] included only partial waves up to
states of formaldehyde, obtained from a three-channel calculation. Portions
of this calculation were done on the Mark IIIfp, the iPSC/860, and the Delta.
Experimental data for these excitations are not available, but an independent
calculation at a similar level of theory has been reported
[Rescigno:90a], and is shown in the figure. Since the complex-Kohn
calculation of [Rescigno:90a] included only partial waves up to
 , we show both the full SMC result, obtained from
, we show both the full SMC result, obtained from 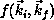 , and a restricted SMC result, obtained with f projected onto a
spherical-harmonic basis
, and a restricted SMC result, obtained with f projected onto a
spherical-harmonic basis 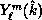 ,
, 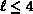 . The agreement
between the restricted SMC result and that of [Rescigno:90a] is in
general excellent; however, comparison to the full SMC result indicates
that such a restriction introduces some errors at higher energies.
. The agreement
between the restricted SMC result and that of [Rescigno:90a] is in
general excellent; however, comparison to the full SMC result indicates
that such a restriction introduces some errors at higher energies.
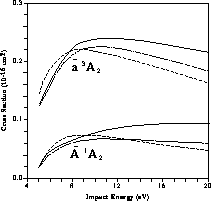
Figure 8.13: Calculated Integral Cross Sections for Electron-Impact Excitation of
the 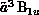 and
and 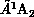 States of
Formaldehyde, Obtained from Three-Channel Calculations. Solid lines:
present SMC results; short-dashed lines: SMC results, limited to
States of
Formaldehyde, Obtained from Three-Channel Calculations. Solid lines:
present SMC results; short-dashed lines: SMC results, limited to
 ; long-dashed lines: complex-Kohn calculations of Ref.\
[Rescigno:90a].
; long-dashed lines: complex-Kohn calculations of Ref.\
[Rescigno:90a].




Next: 8.3.6 Conclusion
Up: Studies of Electron-Molecule
Previous: Intel Machines
Guy Robinson
Wed Mar 1 10:19:35 EST 1995
 H
H ), ethane
(C
), ethane
(C H
H ), propane (C
), propane (C H
H ), disilane (Si
), disilane (Si H
H ), germane
(GeH
), germane
(GeH ), and tetrafluorosilane (SiF
), and tetrafluorosilane (SiF ). We have since studied elastic
scattering by other systems, including phosphine (PH
). We have since studied elastic
scattering by other systems, including phosphine (PH ), propylene
(C
), propylene
(C H
H ) and its isomer cyclopropane, n-butane (C
) and its isomer cyclopropane, n-butane (C H
H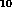 ), and
1,2-trans-difluoroethylene, both on the Mark IIIfp and on the Intel
machines. We have also examined inelastic collisions with ethylene
[Sun:92a], formaldehyde (CH
), and
1,2-trans-difluoroethylene, both on the Mark IIIfp and on the Intel
machines. We have also examined inelastic collisions with ethylene
[Sun:92a], formaldehyde (CH O), methane (CH
O), methane (CH ), and silane
(SiH
), and silane
(SiH ). Below we present selected results of these calculations, where
possible comparing to experimental data.
). Below we present selected results of these calculations, where
possible comparing to experimental data.




 H
H isomers, cyclopropane
and propylene. Scattering from propylene requires some special
consideration, because of its small dipole moment [
isomers, cyclopropane
and propylene. Scattering from propylene requires some special
consideration, because of its small dipole moment [
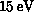 Electrons by Disilane and Ethane. Experimental points for ethane (circles)
are from Ref. [Tanaka:88a]; disilane data (squares) are from Ref.
[Tanaka:89a].
Electrons by Disilane and Ethane. Experimental points for ethane (circles)
are from Ref. [Tanaka:88a]; disilane data (squares) are from Ref.
[Tanaka:89a].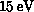 electrons from
ethane and its analogue disilane. These results were obtained on the
Mark IIIfp within the static-approximation. Agreement with experiment
[Tanaka:88a;89a], is quite
good; although there are quantitative differences where the magnitude
of the cross section is small, the qualitative features are well
reproduced for both molecules.
electrons from
ethane and its analogue disilane. These results were obtained on the
Mark IIIfp within the static-approximation. Agreement with experiment
[Tanaka:88a;89a], is quite
good; although there are quantitative differences where the magnitude
of the cross section is small, the qualitative features are well
reproduced for both molecules.
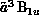 state of ethylene
[
state of ethylene
[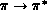 excitation weakens the C-C bond, and its cross
section is relevant to the dissociation of ethylene by low-energy electron
impact. As seen in the figure, the cross section increases rapidly from
threshold (experimental value
excitation weakens the C-C bond, and its cross
section is relevant to the dissociation of ethylene by low-energy electron
impact. As seen in the figure, the cross section increases rapidly from
threshold (experimental value 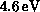 ) and reaches a fairly high peak
value before beginning a gradual decline. The threshold rise is largely due
to a d-wave (
) and reaches a fairly high peak
value before beginning a gradual decline. The threshold rise is largely due
to a d-wave (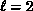 ) contribution, seen as a shoulder around
) contribution, seen as a shoulder around
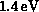 above threshold, which may arise from a core-excited shape
resonance. Relative measurements of this cross section [
above threshold, which may arise from a core-excited shape
resonance. Relative measurements of this cross section [
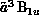 State of Ethylene. Solid line: present
two-channel result; dashed line: relative measurement of Ref. [Veen:76a],
normalized to the calculated value at the broad maximum.
State of Ethylene. Solid line: present
two-channel result; dashed line: relative measurement of Ref. [Veen:76a],
normalized to the calculated value at the broad maximum.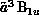 and
and 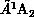 states of formaldehyde, obtained from a three-channel calculation. Portions
of this calculation were done on the Mark IIIfp, the iPSC/860, and the Delta.
Experimental data for these excitations are not available, but an independent
calculation at a similar level of theory has been reported
[
states of formaldehyde, obtained from a three-channel calculation. Portions
of this calculation were done on the Mark IIIfp, the iPSC/860, and the Delta.
Experimental data for these excitations are not available, but an independent
calculation at a similar level of theory has been reported
[ , we show both the full SMC result, obtained from
, we show both the full SMC result, obtained from 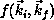 , and a restricted SMC result, obtained with f projected onto a
spherical-harmonic basis
, and a restricted SMC result, obtained with f projected onto a
spherical-harmonic basis 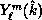 ,
, 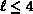 . The agreement
between the restricted SMC result and that of [
. The agreement
between the restricted SMC result and that of [
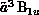 and
and 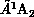 States of
Formaldehyde, Obtained from Three-Channel Calculations. Solid lines:
present SMC results; short-dashed lines: SMC results, limited to
States of
Formaldehyde, Obtained from Three-Channel Calculations. Solid lines:
present SMC results; short-dashed lines: SMC results, limited to
 ; long-dashed lines: complex-Kohn calculations of Ref.\
[Rescigno:90a].
; long-dashed lines: complex-Kohn calculations of Ref.\
[Rescigno:90a].